Regenerative Medicine Market Size, Share, Growth & Industry Analysis, By Product Type (Cell Therapy, Gene Therapy, Tissue Engineering, Small Molecules and Biologics), By Application (Musculoskeletal Disorders, Oncology, Dermatology, Cardiovascular, Neurology, Others), By End-User (Hospitals and Clinics, Research Institutes, Academic Institutes, Specialty Centers), and Regional Analysis, 2024-2031
Regenerative Medicine Market: Global Share and Growth Trajectory
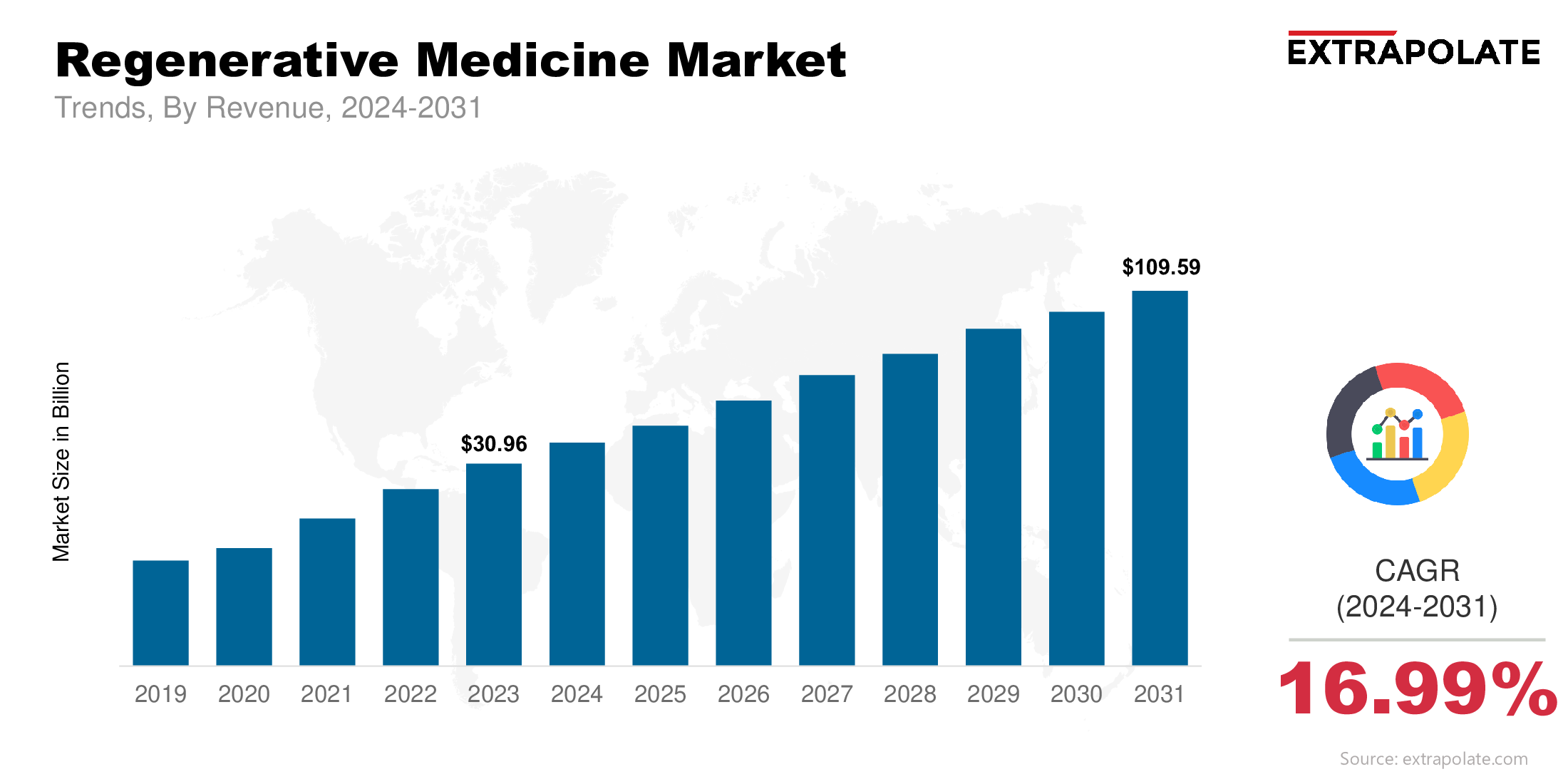
The global Regenerative Medicine Market size was valued at USD 30.96 billion in 2023 and is projected to grow from USD 36.53 billion in 2024 to USD 109.59 billion by 2031, exhibiting a CAGR of 16.9% during the forecast period.
The global market is experiencing remarkable growth as healthcare systems pivot toward curative and personalized treatment solutions. Regenerative medicine, which includes cell therapy, gene therapy, tissue engineering, and stem cell-based approaches, is redefining how chronic diseases, trauma, and degenerative conditions are treated. The market’s momentum is being driven by the increasing prevalence of age-related disorders, a global rise in chronic illnesses, and significant advancements in biotechnological innovations.
What makes regenerative medicine unique is its ability to restore normal body functions. Instead of just managing symptoms, it aims to offer long-term or even permanent solutions. This game-changing potential is drawing major investments. Big pharmaceutical companies, venture capital firms, and healthcare organizations are all getting involved. Also, new advances in CRISPR gene editing, 3D bioprinting, and stem cell therapies are pushing the limits of what can be done in medicine.
Additionally, the trend toward customized treatment and value-based care is influencing the industry. More regenerative medicines are gaining regulatory approval. These medicines are also demonstrating efficacy in practical applications. Adoption is therefore probably set to increase rapidly. Increased product introductions and additional clinical trials highlight the progress in regenerative medicine. Strategic alliances further show its shift from research to routine clinical application.
Key Market Trends Driving Product Adoption
Many key trends are shaping the regenerative medicine field. Also, they are boosting its use in healthcare systems:
- Advances in Stem Cell Therapies: Regenerative medicine still relies heavily on stem cell therapy. New therapeutic possibilities are being made possible by research on induced pluripotent stem cells (iPSCs), adult, and embryonic stem cells. The results of transplants for autoimmune disorders, heart conditions, and spinal cord injuries have improved. This is due to enhanced differentiation techniques and scaffold-based delivery.
- Growth in Gene Therapy Solutions: The application of gene therapy is progressing from theory to practice. Editing and correcting defective genes has become simpler because of tools like CRISPR. Treatments for genetic disorders like hemophilia, cystic fibrosis, and certain types of inherited blindness are becoming possible due to these advancements.
- Expanding Applications in Tissue Engineering: Tissue engineering is now seeing real-world success beyond labs. Scientists can now create tissues for skin grafts, cartilage repair, and even organ frameworks. 3D bioprinting and new biocompatible materials are making organ replacement more achievable.
- Personalized and Precision Medicine:
Regenerative medicine is closely linked with personalized care. Doctors can now tailor therapies based on a patient’s genetic makeup, improving results and reducing side effects. AI tools and biomarker analysis are also helping fine-tune these personalized treatments.
Major Players and their Competitive Positioning
The regenerative medicine industry is highly competitive and innovation-driven. Leading companies are investing in R&D, strategic partnerships, and acquisitions to strengthen their portfolios. Key players in this evolving landscape includeOrganogenesis Holdings Inc., Novartis AG, Vericel Corporation, Smith & Nephew PLC, Integra LifeSciences Holdings Corporation, Astellas Pharma Inc., Takeda Pharmaceutical Company Limited, Medipost Co., Ltd., MiMedx Group, Inc., Thermo Fisher Scientific Inc and others.
These firms are investing in clinical trials, forming academic-industry collaborations, and developing scalable manufacturing techniques to meet rising global demand.
Consumer Behavior Analysis
Consumer interest in regenerative therapies is shaped by awareness, cost, and treatment results:
- Demand for Curative Therapies: Nowadays, many people are searching for long-term fixes rather than only symptom management. Chronic illnesses may be permanently cured or made better by regenerative therapy. They are therefore highly appealing.
- Acceptance of New Therapies: As more people learn about stem cells and gene editing, they are becoming more open to regenerative medicine. Awareness campaigns and clear clinical trial results are helping build trust and reduce doubts.
- Weighing Cost and Benefit: These treatments can be expensive at first. However, many patients and healthcare providers see the long-term value. They can lead to fewer hospital visits, a better quality of life, and quicker recovery times.
- Growth in Medical Tourism: In developed countries, high costs and limited access are pushing patients to seek treatment abroad. Emerging markets often offer these therapies at lower prices with fewer regulatory delays. This shift is changing how and where people access regenerative care.
Pricing Trends
The type of therapy, medical indication, and delivery technique all affect pricing in the market. Advanced treatments like gene editing and autologous stem cell therapy can run into the tens of thousands of dollars. Costs are, nevertheless, progressively going down. Automation and mass production methods are lowering the cost of scaffold-based and allogeneic medicines.
Bioreactor leasing and pay-per-use tissue engineering platform models are also growing in popularity. Reimbursement schemes for FDA-approved goods are also becoming more popular. Regenerative medicines are becoming more accessible as a result of these advancements.
Growth Factors
The market is growing fast. Several key factors are driving this upward trend:
- Technological Breakthroughs: Research on stem cells, gene editing, and tissue engineering have all advanced quickly. New therapy options are being made possible by technologies like induced pluripotent stem cells, 3D bioprinting, and CRISPR. The way complicated and previously incurable illnesses were being treated is evolving as a result of these technologies.
- Rise in Chronic and Degenerative Diseases: Osteoarthritis, diabetes, and heart disease are among the conditions that are on the rise. Treatments that can replace or repair damaged tissues are now more necessary as a result. As the population ages, the demand is much greater, particularly in North America, Europe, and some regions of Asia.
- Government and Institutional Support: Many governments are actively supporting regenerative medicine. They are providing research funding, faster approvals, and regulatory help. For example, the U.S. FDA’s RMAT designation and similar programs in Japan and the EU are speeding up the approval of new treatments.
- Growing Investments and M&A Activities: There’s been a sharp rise in venture capital funding and mergers and acquisitions in this field. Companies are using these deals to access advanced technologies, strengthen their research pipelines, and enter new markets.
Regulatory Landscape
Rapid innovation is causing the regulatory environment in regenerative medicine to change.
- Faster paths like RMAT designation have been proposed by the FDA in the United States. Additionally, it provides accelerated reviews for potential gene and cell therapies.
- Regenerative products are classified as Advanced Therapy Medicinal Products (ATMPs) by the European Medicines Agency (EMA) in Europe. A centralized approval process is used to these products.
- Japan has a progressive system. After early-phase trials, it permits regenerative medicines to receive conditional approval.
In addition to encouraging innovation and clinical acceptance, these rules seek to protect patient safety.
Recent Developments
Recent developments highlight the rapid progress in the regenerative medicine industry:
- Vertex Pharmaceuticals and CRISPR Therapeutics reported successful late-stage trials. Their gene-editing therapies target sickle cell disease and beta-thalassemia.
- Organogenesis launched new wound care products using cryopreserved amniotic tissue. These products have shown positive results in treating chronic ulcers.
- Vericel Corporation expanded its autologous chondrocyte implantation therapy. It is now being used more widely to treat knee cartilage defects and is gaining acceptance among orthopedic specialists.
- Collaborations between academic institutions and biotech firms are increasing. Harvard’s Wyss Institute, for example, is partnering with startups to develop bioengineered organs.
These updates reflect growing clinical confidence, steady innovation, and an expanding range of treatment options.
Current and Potential Growth Implications
a. Demand-Supply Analysis
The demand for regenerative therapies is rising quickly. However, current supply levels, especially in cell therapy, are not sufficient. Scaling up cell manufacturing has become a key industry focus. Maintaining quality and controlling costs are equally important. Advanced biomanufacturing platforms are supporting this growth. Closed-system production lines are also helping meet the demand.
b. Gap Analysis
Although the industry is growing, significant gaps still exist. Among these are a lack of uniformity, ambiguous laws, and low public awareness. Cooperation between researchers, companies, and regulators will be necessary to address these issues. Equal access must also be guaranteed in both high- and low-income areas.
Top Companies in the Regenerative Medicine Market
Key players in the regenerative medicine industry include:
- Organogenesis Holdings Inc.
- Novartis AG
- Vericel Corporation
- Smith & Nephew PLC
- Integra LifeSciences Holdings Corporation
- Astellas Pharma Inc.
- Takeda Pharmaceutical Company Limited
- Medipost Co., Ltd.
- MiMedx Group, Inc.
- Thermo Fisher Scientific Inc.
These firms are continuously investing in R&D, clinical validation, and global expansion to sustain their leadership in the market.
Regenerative Medicine Market: Report Snapshot
Segmentation | Details |
By Product Type | Cell Therapy, Gene Therapy, Tissue Engineering, Small Molecules and Biologics |
By Application | Musculoskeletal Disorders, Oncology, Dermatology, Cardiovascular, Neurology, Others |
By End-User | Hospitals and Clinics, Research Institutes, Academic Institutes, Specialty Centers |
By Region | North America, Europe, Asia-Pacific, Latin America, Middle East & Africa |
High Growth Segments
- Cell Therapy: Cell therapy is the largest and fastest-growing segment. It is used in oncology, autoimmune disorders, and musculoskeletal conditions. Advances in stem cell manufacturing and quality control are improving scalability and clinical use.
- Gene Therapy: Gene therapy is expected to grow rapidly. This is driven by increasing regulatory approvals and successful clinical trials. It shows strong potential in treating rare diseases.
- Tissue Engineering: Demand is rising for engineered tissues, especially for skin, cartilage, and vascular use. This is encouraging progress in scaffold materials and bioprinting technologies.
Major Innovations
Notable innovations in the regenerative medicine sector include:
- CRISPR-based Gene Editing: This technology enables precise correction of faulty genes. It is especially useful for treating inherited disorders.
- 3D Bioprinting: It allows the creation of tissues with blood vessels. Organ-like structures can also be developed using this method.
- Exosome-based Therapies: These therapies support communication between cells. They also promote natural repair processes.
- Biodegradable Scaffolds: These are designed to help tissue grow. They gradually dissolve without causing side effects.
These advancements are driving the development of next-generation regenerative therapies tailored to individual needs.
Potential Growth Opportunities
- Expansion in Emerging Economies: Countries in Asia-Pacific and Latin America are investing more in healthcare and biotech research. Supportive policies and rising disease rates make these regions ideal for market growth.
- Integration with AI and Digital Health: Regenerative medicine is now using AI, machine learning, and big data. These tools help with faster decisions, better predictions, and patient targeting. Telemedicine also supports remote monitoring and follow-ups.
Extrapolate Research Says
The regenerative medicine market is entering a significant phase of growth. Innovation, clinical adoption, and commercial potential are advancing rapidly. With the integration of biotechnology, artificial intelligence, and personalized healthcare, regenerative therapies are no longer seen as futuristic. They are now practical solutions improving clinical outcomes.
According to Kings Research, the market is expected to grow steadily. This growth is driven by the increasing prevalence of chronic and degenerative diseases, faster regulatory approvals, and rising global investments. Companies are now working toward scalable manufacturing and standardized treatment protocols. As a result, regenerative medicine is likely to expand from niche applications to broader use across various medical specialties.
ARE YOU SEEKING COMPREHENSIVE INSIGHT ON VARIOUS
MARKETS?
CONTACT OUR EXPERTS TODAY

Regenerative Medicine Market Size
- June-2025
- 148
- Global
- healthcare-medical-devices-biotechnology
Related Research
2022-2030 Global and Regional B-Cell Non-Hodgkin`s Lymphoma (NHL) Treatment Industry Production, Sa
February-2021
2022-2030 Global and Regional Organ Transplant Diagnostics Industry Production, Sales and Consumpti
February-2021
2022-2030 Global and Regional Prostacyclin Drug Industry Production, Sales and Consumption Status a
February-2021
2022-2030 Global and Regional Spinal and Bulbar Muscular Atrophy Treatment Industry Production, Sal
February-2021
2% Chlorhexidine Gluconate (CHG) Cloths-Global Market Status & Trend Report 2022-2030 Top 20 Countri
April-2021
2021-2027 Global and RegioAnal Nasal Gels Industry Production, Sales and Consumption Status and Pros
February-2021
2021-2027 Global and Regional 2019-nCoV Detection Server Industry Production, Sales and Consumption
February-2021
2021-2027 Global and Regional ? Collagen Quantitative Determination Kit Industry Production, Sales a
February-2021
2021-2027 Global and Regional Anti-TNF Monoclonal Antibody Industry Production, Sales and Consumptio
February-2021